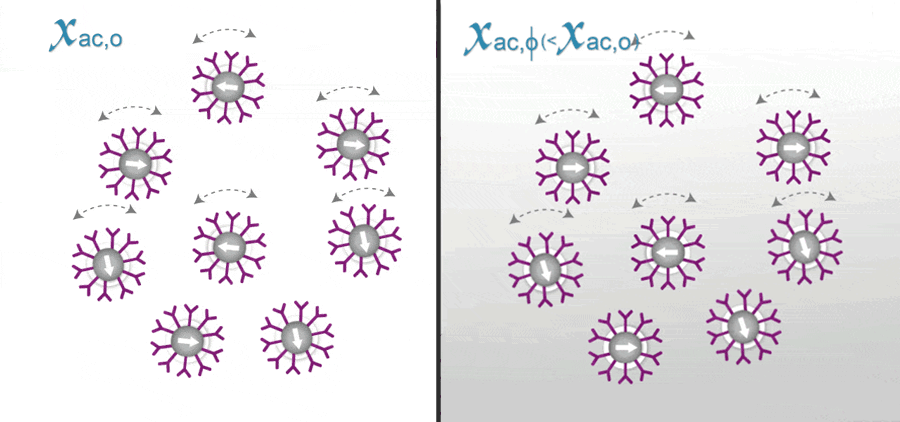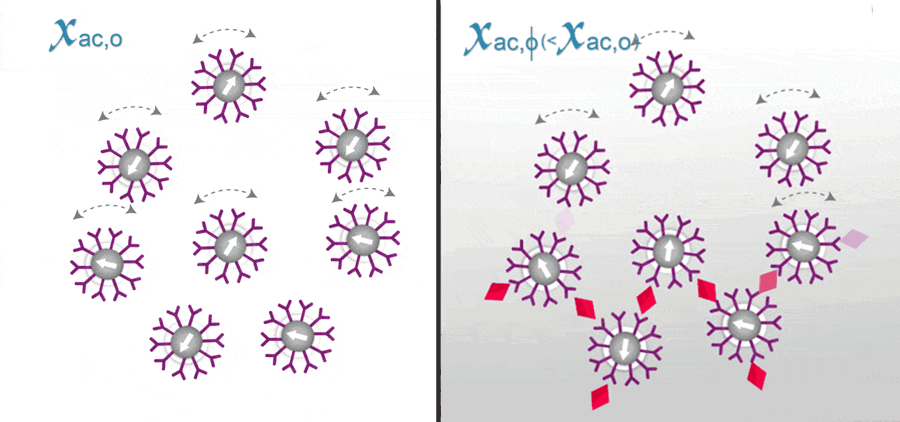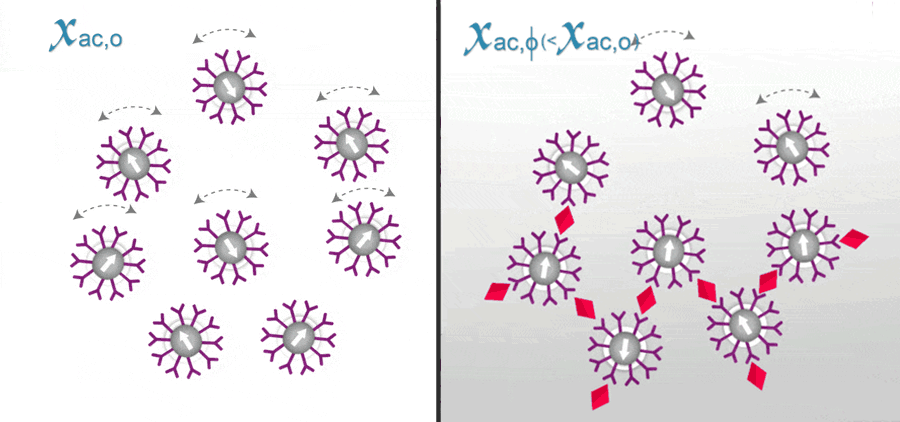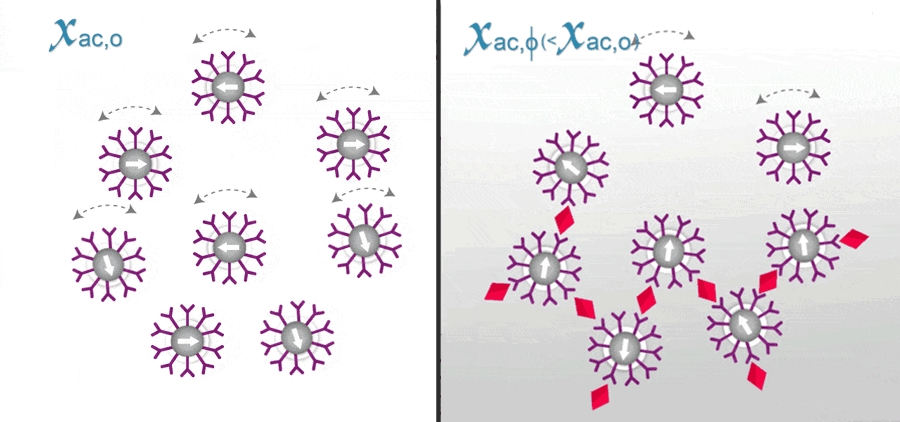
Mechanism
In ImmunoMagnetic Reduction (IMR), the reagent is a solution having homogeneously dispersed magnetic nanoparticles, which are coated with hydrophilic surfactants and bio-probe (e.g. antibodies). Under external multiple ac magnetic fields, magnetic nanoparticles oscillate with the multiple ac magnetic fields via magnetic interaction. Thus, the reagent under external multiple ac magnetic fields shows a magnetic property, called mixed-frequency ac magnetic susceptibility Xac. Via the bio-probes on the outmost shell, magnetic nanoparticles associate with and magnetically label bio-molecules (e.g. antigens) to be detected. Due to the association, magnetic nanoparticles become either larger, as schematically shown.
The response of these larger magnetic nanoparticles to external multiple ac magnetic fields is much less than that of originally individual magnetic nanoparticles. Thus, the Xac of the reagent is reduced due to the association between magnetic nanoparticles and detected bio-molecules. This is why the method is referred as ImmunoMagnetic Reduction. In principle, when more amounts of to-be-detected bio-molecules are mixed with a reagent, more magnetic nanoparticles become larger. A larger reduction in Xac could be expected for reagents.
Merits
According to the description given above, IMR exhibits several unique merits, as described below.
Firstly, the unbound to-be-detected bio-molecules and magnetic nanoparticles are not necessarily to be removed. They are still in the reagent. So, the assay process of IMR is simple because of wash-free processes.
Secondly, only one kind of bio-probe is used.
Thirdly, IMR is a direct and homogeneous assay, which usually shows high reliability and sensitivity. Especially, magnetic nanoparticles show high surface-to-volume ratio. The binding area between antibodies and to-be-detected bio-molecules is extremely large, which results in a high sensitivity.
Fourthly, the non-specific binding between the bio-probes and non-target bio-molecules is highly suppressed. The mechanism for the high suppression in non-specific binding is briefly explained as follows. Magnetic nanoparticles oscillate with the external ac magnetic fields when detecting the χac of the magnetic reagent. Both target and non-target bio-molecules bound with bio-probes on the oscillating magnetic nanoparticles experience centrifugal forces. At high oscillating frequencies, the centrifugal force is enhanced. Once the centrifugal force is stronger than the binding force between the bio-probes and non-target bio-molecules, the nonspecific binding is broken down. However, the centrifugal force must be weaker than the specific binding force between bio-probes and to-be-detected bio-molecules. As a result, the cross reactions are inhibited for IMR.
Fifthly, because the amount of reduction in χac can be accurately measured to correspond to the concentration of the to-be-detected bio-molecules, the concentration of the bio-molecules can thus be measured quantitatively.
Several papers have demonstrated that IMR can be applied to assay proteins, viruses, chemicals, and nucleic acids once suitable bio-probes are immobilized onto the magnetic nanoparticles.
Reference(s)
- C.Y. Hong, C.C. Wu, Y.C. Chiu, S.Y. Yang, H.E. Horng, and H.C. Yang, “Magnetic susceptibility reduction method for magnetically labeled immunoassay”, Appl. Phys. Lett. 88, 212512 (2006).
- S.Y. Yang, Z.F. Jian, H.E. Horng, C.Y. Hong, H.C. Yang, C.C. Wu, and Y.H. Lee, “Dual immobilization and magnetic manipulation of magnetic nanoparticles”, J. Magn. Magn. Mater. 320, 2688 (2008).
- S.Y. Yang, J.J. Chieh, W.C. Wang, C.Y. Yu, C.B. Lan, J.H. Chen, H.E. Horng, C.Y. Hong, H.C. Yang, and Wilber Huang, “Ultra-highly sensitive and wash-free bio-detection of H5N1 virus by immunomagnetic reduction assays”, J. Virol. Methods 153 250 (2008).
- S.Y. Yang, J.J. Chieh, W.C. Wang, C.Y. Yu, N.S. Hing, H.E. Horng, C.Y. Hong, H.C. Yang, C.F. Chang, and H.Y. Lin, “Magnetic nanoparticles for high-sensitivity detection on nucleic acids via superconducting-quantum-interference-device-based immunomagnetic reduction assay”, J. Magn. Magn. Mater. 323 681 (2011).
- C.C. Yang, S.Y. Yang, J.J. Chieh, H.E. Horng, C.Y. Hong, and H.C. Yang, “Universal behavior of biomolecule- concentration-dependent reduction in ac magnetic susceptibility of bioreagents”, Magn. Lett. IEEE 3, 1500104 (2012).
- C.C. Yang, S.Y. Yang, H.H. Chen, W.L. Weng, H.E. Horng, J.J. Chieh, C.Y. Hong, and H.C. Yang, “Effect of molecule-particle binding on the reduction in the mixed-frequency ac magnetic susceptibility of magnetic bio-reagents”, J. Appl. Phys. 112, 24704 (2012).
- S.Y. Yang, C.C. Yang, H.E. Horng, B.Y. Shih, J.J. Chieh, C.Y. Hong, and H.C. Yang, “Experimental study on low-detection limit for immunomagnetic reduction assays by manipulating reagent entities”, IEEE Trans. NanoBioSci. 12, 65 (2013).
- S.Y. Yang, J.F. Chang, T.C. Chen, C.C. Yang, and C.S. Ho, “Study of the temperature dependent immuno-reaction kinetics for the bio-functionalized magnetic nanoparticle assay of bio-markers of colorectal cancer”, Appl. Phys. Lett. 104, 013702 (2014).
- “Method of quantitatively measuring biomolecules”, US patent 7560289.
- “Method and system for suppressing bindings on magnetic particles”, US patent 8053250.